Unconventional approaches for the introduction of sulfur-based functional groups
Abstract
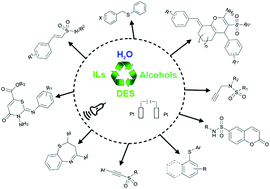
Organosulfur compounds have a pivotal role in the functionalities of many natural products, pharmaceuticals and organic materials. For these reasons, the search for new methodologies for the formation of carbon–sulfur bonds has been the object of intensive work for organic chemists. However, the proposed strategies suffer from various drawbacks, such as volatility, toxicity, and instability of the sulfur sources or the use of VOC solvents. In this review, we summarise the recent protocols which have the goal of obtaining sulfones, thioethers, thiazines, thiazepines and sulfonamides in an unconventional and/or sustainable way. The use of starting materials less invasive and toxic with respect to the traditional reagents, alternative solvents such as water, ionic liquids or deep eutectic solvents, the exploitation of ultrasound and electrochemistry, increasing the efficiency of the process, are reported. Moreover, representative reaction mechanisms are also discussed.
- This article is part of the themed collection: Synthetic methodology in OBC

 Please wait while we load your content...
Please wait while we load your content...