Chalcogen bonding mediates the formation of supramolecular helices of azapeptides in crystals†
Abstract
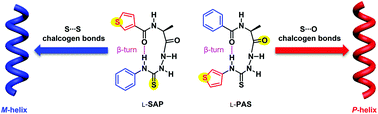
To explore whether chalcogen bonding was able to drive the formation of supramolecular helices, alanine-based azapeptides containing a β-turn structure, with a thiophene group, respectively, incorporated in the N- or C-terminus, were employed as helical building blocks. While the former derivative formed a supramolecular M-helix via intermolecular S⋯S chalcogen bonding in crystals, the latter formed P-helix via intermolecular S⋯O chalcogen bonding.
- This article is part of the themed collections: Editor’s Collection and Supramolecular chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...