Fluorescent 4-amino-1,8-naphthalimide Tröger's bases (TBNaps) possessing (orthogonal) ‘α-amino acids’, esters and di-peptides and their solvent dependent photophysical properties†
Abstract
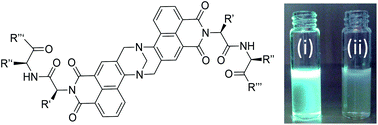
The synthesis of fifteen luminescent bis-naphthalimide based Tröger's bases (TBNaps) derived from 4-amino-1,8-naphthalimide (4-Amino-Nap) precursors is described; these scaffolds possess α-amino acids, esters or di-peptides conjugated at the imide site and show minor fluorescence in aqueous solution while being highly emissive in organic solvents. The investigation shows that these TBNaps possessing ICT excited state properties are capable of generating either positive or negative solvatochromic effects in response to changes in polarity and/or the hydrogen bonding capabilities of the medium.
- This article is part of the themed collection: Supramolecular chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...