A promiscuous glycosyltransferase generates poly-β-1,4-glucan derivatives that facilitate mass spectrometry-based detection of cellulolytic enzymes†
Abstract
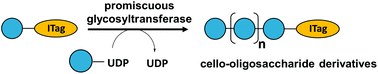
Promiscuous activity of a glycosyltransferase was exploited to polymerise glucose from UDP-glucose via the generation of β-1,4-glycosidic linkages. The biocatalyst was incorporated into biocatalytic cascades and chemo-enzymatic strategies to synthesise cello-oligosaccharides with tailored functionalities on a scale suitable for employment in mass spectrometry-based assays. The resulting glycan structures enabled reporting of the activity and selectivity of celluloltic enzymes.
- This article is part of the themed collections: Chemical Biology in OBC and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...