Alkylation of the α-amino C–H bonds of anilines photocatalyzed by a DMEDA-Cu-benzophenone complex: reaction scope and mechanistic studies†‡
Abstract
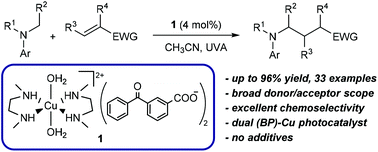
The Cu(II) complex 1 incorporating a BP chromophore is a highly active and chemoselective photocatalyst for the alkylation of α-amino C–H bonds of anilines. The reaction was shown to proceed with a broad substrate scope in the absence of additives. Extensive mechanistic studies were performed, in particular using transient absorption spectroscopy, and spectroscopic signatures of key intermediates were identified in the conditions of catalysis. Finally, the ability of 1 to act as a multitask catalyst was showcased by conducting multi-component CuAAC and olefin hydroalkylation reactions in one-pot.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...