Cycloaddition of cyclopropanes for the elaboration of medium-sized carbocycles
Abstract
The stereocontrolled formation of medium-sized carbocycles is a major goal in modern organic chemistry due to their widespread occurrence in natural products and pharmaceutically active ingredients. One approach consists in the use of cycloaddition reactions which notably results in high selectivities and atom-economy. To this end, cyclopropanes are ideal substrates since they can provide readily functionalized three- or five-carbon synthons. Herein we report advances made in cycloaddition reactions of cyclopropanes towards the synthesis of medium-sized carbocycles via transition metal catalysis or Lewis acid catalysis.
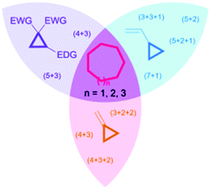
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...