The structure and biosynthesis of heinamides A1–A3 and B1–B5, antifungal members of the laxaphycin lipopeptide family†
Abstract
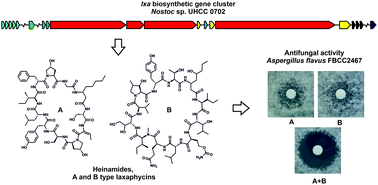
Laxaphycins are a family of cyclic lipopeptides with synergistic antifungal and antiproliferative activities. They are produced by multiple cyanobacterial genera and comprise two sets of structurally unrelated 11- and 12-residue macrocyclic lipopeptides. Here, we report the discovery of new antifungal laxaphycins from Nostoc sp. UHCC 0702, which we name heinamides, through antimicrobial bioactivity screening. We characterized the chemical structures of eight heinamide structural variants A1–A3 and B1–B5. These variants contain the rare non-proteinogenic amino acids 3-hydroxy-4-methylproline, 4-hydroxyproline, 3-hydroxy-D-leucine, dehydrobutyrine, 5-hydroxyl β-amino octanoic acid, and O-carbamoyl-homoserine. We obtained an 8.6-Mb complete genome sequence from Nostoc sp. UHCC 0702 and identified the 93 kb heinamide biosynthetic gene cluster. The structurally distinct heinamides A1–A3 and B1–B5 variants are synthesized using an unusual branching biosynthetic pathway. The heinamide biosynthetic pathway also encodes several enzymes that supply non-proteinogenic amino acids to the heinamide synthetase. Through heterologous expression, we showed that (2S,4R)-4-hydroxy-L-proline is supplied through the action of a novel enzyme LxaN, which hydroxylates L-proline. 11- and 12-residue heinamides have the characteristic synergistic activity of laxaphycins against Aspergillus flavus FBCC 2467. Structural and genetic information of heinamides may prove useful in future discovery of natural products and drug development.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...