Recent developments in asymmetric Heck type cyclization reactions for constructions of complex molecules
Abstract
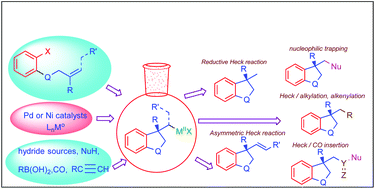
Intramolecular carbometallation-initiated asymmetric transformations are a general and powerful approach for the construction of carbo- and heterocyclic systems with one and more stereocenters. In addition, the newly developed multiple cascade reactions are an attractive strategy for increasing the molecular complexity in one step. In recent years, great progress has been made in this area with the use of various palladium and nickel complexes with P- and N-donor chiral ligands. This review highlights recent developments in intramolecular asymmetric Heck reactions, reductive Heck reactions and various types of cascade transformations (intramolecular Heck/Heck, Heck/nucleophilic trapping, Heck/Tsuji–Trost, Heck/Suzuki–Miyaura, Heck/Sonogashira, and Heck/carbonylation) in the synthesis of complex molecules over the past 5 years. A number of examples from before 2016 are included as background information. Particular attention is paid to the use of inexpensive nickel complexes as highly efficient catalysts for a number of asymmetric reactions considered here. A perspective on current challenges and potential future developments in the field of asymmetric Heck type cyclizations is also provided.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...