A versatile iodo(iii)etherification of terminal ethynylsilanes using BF3–OiPr2 and alkyl benzyl ethers†
Abstract
A series of (E)-α-silyl-β-alkoxyvinyl-λ3-iodanes was synthesized from iodosylbenzene, BF3–ether complexes, and terminal ethynylsilanes. The combined use of BF3–OiPr2 and benzyl ethers of primary alcohols (ROBn) allows the chemoselective transfer of primary alkoxy groups (RO) onto the β-position of the terminal ethynylsilanes.
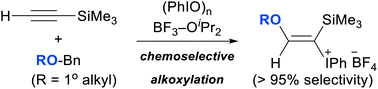
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...