Ag(i)-Catalyzed rapid access to 2-amino-4-methylenethiazolines with potential applications in bioconjugation chemistry†
Abstract
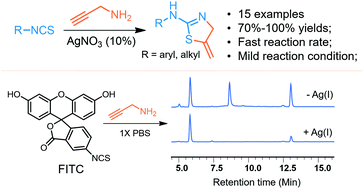
An Ag(I)-catalyzed tandem addition–cyclization of isothiocyanate and propargylamine was successfully applied to the synthesis of 2-amino-4-methylenethiazolines. This route features an unprecedented fast reaction rate with full conversion reached within 10 min at room temperature for aromatic isothiocyanates and excellent chemoselectivity for exocyclic products. The application of this strategy is further highlighted by the accelerated bioconjugation of propargylamine with fluorescein isothiocyanate (FITC) under Ag(I)-catalysis.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...