Assembly and optical properties of 1D helical bundles induced by triphenylamine, side chains and solvents in crystals†
Abstract
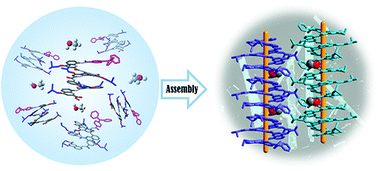
Two novel helical aromatic foldamer derivatives TPA-Q6(n-He) and TPA-Q6(i-Bu) were synthesized and characterized by introducing n-hexyloxy and isobutoxy side chains, respectively, and modifying quinoline amide foldamers with the triphenylamine (TPA) moiety at the N-terminus. X-ray single crystal diffraction analyses and theoretical calculations showed that the quinoline amide hexamer derivative TPA-Q6(i-Bu) enabled one-dimensional (1D) helical self-assembly in solids due to the synergistic interaction of the flexible π units of TPA, the steric hindrance of the alkyl groups, and methanol molecules. The chemical modification of the TPA end group significantly enhanced the fluorescence due to the intramolecular charge transfer. Steady-state fluorescence spectra and transient decay curves showed that TPA-Q6(i-Bu) forming a 1D helical assembly obviously exhibited a redshift of the emission wavelength and an increase of phosphorescence lifetimes in crystals compared with TPA-Q6(n-He) adopting the alternating insertion arrangement induced by the long alkyl chains. The results offered a new way to explore the intrinsic relationship among molecular structures, packing modes and optical properties for foldamers.
- This article is part of the themed collection: Supramolecular chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...