Amidino substituted 2-aminophenols: biologically important building blocks for the amidino-functionalization of 2-substituted benzoxazoles†
Abstract
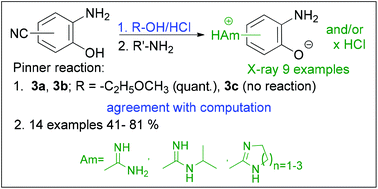
Unlike the closely related and widely investigated amidino-substituted benzimidazoles and benzothiazoles with a range of demonstrated biological activities, the matching benzoxazole analogues still remain a largely understudied and not systematically evaluated class of compounds. To address this challenge, we utilized the Pinner reaction to convert isomeric cyano-substituted 2-aminophenols into their amidine derivatives, which were isolated as hydrochlorides and/or zwitterions, and whose structure was confirmed by single crystal X-ray diffraction. The key step during the Pinner synthesis of the crucial carboximidate intermediates was characterized through mechanistic DFT calculations, with the obtained kinetic and thermodynamic parameters indicating full agreement with the experimental observations. The obtained amidines were subjected to a condensation reaction with aryl carboxylic acids that allowed the synthesis of a new library of 5- and 6-amidino substituted 2-arylbenzoxazoles. Their antiproliferative features against four human tumour cell lines (SW620, HepG2, CFPAC-1, HeLa) revealed sub-micromolar activities on SW620 for several cyclic amidino 2-naphthyl benzoxazoles, thus demonstrating the usefulness of the proposed synthetic strategy and promoting amidino substituted 2-aminophenols as important building blocks towards biologically active systems.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...