Metal-free access to 3-allyl-2-alkoxychromanones via phosphine-catalyzed alkoxy allylation of chromones with MBH carbonates and alcohols†
Abstract
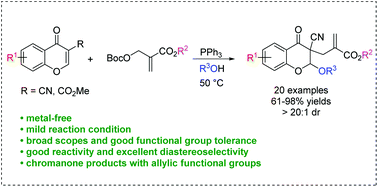
A metal-free access to 3-allyl-2-alkoxychromanones by PPh3-catalyzed alkoxy allylation of chromones with MBH carbonates and alcohols is described. This reaction is performed under mild conditions and it shows good functional group tolerance, providing a series of functionalized chromanones in moderate to high yields with excellent diastereoselectivities. Deuterium-labeling experiments to probe a possible mechanism and scale-up reaction were also conducted.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...