Nickel-catalyzed coupling of R2P(O)Me (R = aryl or alkoxy) with (hetero)arylmethyl alcohols†
Abstract
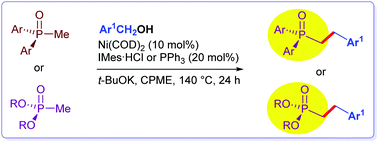
α-Alkylation of methyldiarylphosphine oxides with (hetero)arylmethyl alcohols was performed under nickel catalysis. Various arylmethyl and heteroarylmethyl alcohols can be used in this transformation. A series of methyldiarylphosphine oxides were alkylated with 30–90% yields. Functional groups on the aromatic rings of methyldiarylphosphine oxides or arylmethyl alcohols including OMe, NMe2, SMe, CF3, Cl, and F groups can be tolerated. The conditions are also suitable for the α-alkylation reaction of dialkyl methylphosphonates.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...