Stereocontrolled syntheses of (−)- and (+)-γ-diisoeugenol along with optically active eight stereoisomers of 7,8′-epoxy-8,7′-neolignan†
Abstract
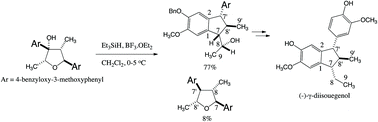
It was shown that reduction of the tertiary benzylic hydroxy group of (2R,3S,4R,5S)-3,5-bis(4-benzyloxy-3-methoxyphenyl)-2,4-dimethyltetrahydro-3-furanol 17 followed by the intramolecular Friedel–Crafts reaction gave exclusively indane with (7S,7′S,8R,8′R)-2,7′-cyclo-7,8′-neolignan structure 18 along with (7S,7′R,8S,8′R)-7,8′-epoxy-8,7′-neolignan structure 19. Indane 18 was converted to (−)-γ-diisoeugenol ((−)-4). On the other hand, (2S,3R,4R,5S)-3,5-bis(4-benzyloxy-3-methoxyphenyl)-2,4-dimethyltetrahydro-3-furanol 22 did not afford indane, but the tetrahydrofuran structure with (7S,7′S,8S,8′S)-7,8′-epoxy-8,7′-neolignan structure 23 and 7′-epi-23.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...