Synthesis of multi-substituted pyridines from ylidenemalononitriles and their emission properties†
Abstract
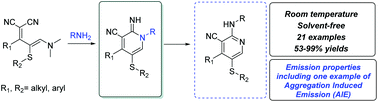
Numerous methodologies to obtain pyridines from ylidenemalononitriles are described in the literature. Nevertheless, they are limited to the use of microwave or conventional heat and few lead to 2,3,4 or 2,3,4,5-substituted pyridines as multi-proposal molecular scaffolds or even universal pyridines. Herein, we present a mild and facile solvent-free methodology to obtain a scope of multi-substituted pyridines at room temperature. We also report an example where one of the resulting amino-nicotinonitriles exhibits a preliminary evidence of aggregation-induced emission (AIE).
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...