PhI(OAc)2 and iodine-mediated synthesis of N-alkyl sulfonamides derived from polycyclic aromatic hydrocarbon scaffolds and determination of their antibacterial and cytotoxic activities†
Abstract
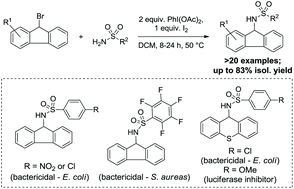
The development of new approaches toward chemo- and regioselective functionalization of polycyclic aromatic hydrocarbon (PAH) scaffolds will provide opportunities for the synthesis of novel biologically active small molecules that exploit the high degree of lipophilicity imparted by the PAH unit. Herein, we report a new synthetic method for C–X bond substitution that is speculated to operate via a N-centered radical (NCR) mechanism according to experimental observations. A series of PAH sulfonamides have been synthesized and their biological activity has been evaluated against Gram-negative and Gram-positive bacterial strains (using a BacTiter-Glo assay) along with a series of mammalian cell lines (using CellTiter-Blue and CellTiter-Glo assays). The viability assays have resulted in the discovery of a number of bactericidal compounds that exhibit potency similar to other well-known antibacterials such as kanamycin and tetracycline, along with the discovery of a luciferase inhibitor. Additionally, the physicochemical and drug-likeness properties of the compounds were determined experimentally and using in silico approaches and the results are presented and discussed within.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...