Hydroxyl-group-activated azomethine ylides in organocatalytic H-bond-assisted 1,3-dipolar cycloadditions and beyond
Abstract
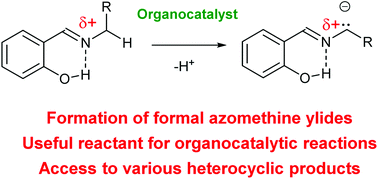
1,3-Dipolar cycloaddition constitutes a powerful means for the synthesis of five-membered heterocycles. Recently, the potential of this field of chemistry has been expanded by the employment of organocatalytic activation strategies. One group of substrates, namely imines derived from salicylaldehydes, is particularly useful. Additional activation via intramolecular H-bonding interactions offered by the presence of an ortho-hydroxyl phenolic group in their structure results in increased reactivity of these reactants. Furthermore, it can be utilized in subsequent reactions creating chemical and stereochemical diversity. This minireview provides a summary of the recent progress in this field of organocatalysis and indicates other important applications of hydroxyl-group-activated azomethine ylides in asymmetric organocatalysis.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...