Chemoselective synthesis of fully substituted pyrroles via a one-pot four-component isocyanide-based reaction†
Abstract
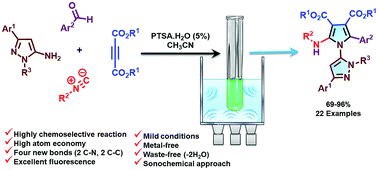
Small-ring heterocycles comprising pyrrole and pyrazole are well known for their rich biological properties. In this article, an efficient green sonochemical approach was designed for the synthesis of novel, fully substituted pyrroles connected to pyrazole scaffolds via a one-pot, four-component isocyanide-based sequential reaction. This reaction was carried out using various 5-amino-pyrazoles, aldehydes, dialkyl acetylenedicarboxylates and isocyanides for the synthesis of fully functionalized pyrroles with high chemoselectivity in the presence of a catalytic amount of PTSA·H2O, in good to excellent yields under ultrasound irradiation. This waste-free (–H2O) reaction exhibited a high atom economy and step economy via creating four new bonds, including two C–N and two C–C bonds, and the formation of two five-member heterocycles which are connected in a single operation. The mechanism of this four-component domino process involved sequential imination-dipolar cyclization-[1,5]-H shift reactions. The synthesized compounds possess interesting fluorescence features, and the bioactive scaffolds might attract great interest in the fields of clinical diagnostics and biomedical research in the future.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...