Kinetic and structure–activity studies of the triazolium ion-catalysed benzoin condensation†
Abstract
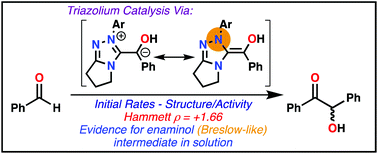
Steady-state kinetic and structure–activity studies of a series of six triazolium-ion pre-catalysts 2a–2f were investigated for the benzoin condensation. These data provide quantitative insight into the role of triazolium N-aryl substitution under synthetically relevant catalytic conditions in a polar solvent environment. Kinetic behaviour was significantly different to that previously reported for a related thiazolium-ion pre-catalyst 1, with the observed levelling of initial rate constants to νmax at high aldehyde concentrations for all triazolium catalysts. Values for νmax for 2a–2f increase with electron withdrawing N-aryl substituents, in agreement with reported optimal synthetic outcomes under catalytic conditions, and vary by 75-fold across the series. The levelling of rate constants supports a change in rate-limiting step and evidence supports the assignment of the Breslow-intermediate forming step to the plateau region. Correlation of νmax reaction data yielded a positive Hammett ρ-value (ρ = +1.66) supporting the build up of electron density adjacent to the triazolium N-Ar in the rate-limiting step favoured by electron withdrawing N-aryl substituents. At lower concentrations of aldehyde, both Breslow-intermediate and benzoin formation are partially rate-limiting.
- This article is part of the themed collection: Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...