A mild and chemoselective CALB biocatalysed synthesis of sulfoxides exploiting the dual role of AcOEt as solvent and reagent†
Abstract
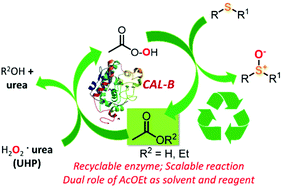
A mild, chemoselective and sustainable biocatalysed synthesis of sulfoxides has been developed exploiting CALB and using AcOEt with a dual role of more environmentally friendly reaction solvent and enzyme substrate. A series of sulfoxides, including the drug omeprazole, have been synthesised in high yields and with excellent E-factors.
- This article is part of the themed collections: Biocatalysis: A cross-journal collection and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...