Suppressing vanadium dissolution of V2O5via in situ polyethylene glycol intercalation towards ultralong lifetime room/low-temperature zinc-ion batteries†
Abstract
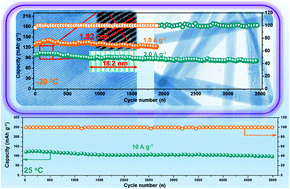
Zinc-ion batteries (ZIBs) are a main focus worldwide for their potential use in large-scale energy storage due to their abundant resources, environmental friendliness, and high safety. However, the cathode materials of ZIBs are limited, requiring a stable host structure and fast Zn2+ channel diffusion. Here, we develop a strategy for the intercalation of polyethylene glycol (PEG) to facilitate Zn2+ intercalation and to suppress the dissolution of vanadium in V2O5. In particular, PEG-V2O5 shows a high capacity of 430 mA h g−1 at a current density of 0.1 A g−1 as well as excellent 100 mA h g−1 specific capacity after 5000 cycles, with a high current density of 10.0 A g−1. A reversible capacity of 81 mA h g−1 can even be achieved with a low temperature of −20 °C at a current density of 2.0 A g−1 after 3500 cycles. The superior electrochemical performance comes from the intercalation of PEG molecules, which can improve kinetic transport and structural stability during the cycling process. The Zn2+ storage mechanism, which provides essential guidelines for the development of high-performance ZIBs, can be found through various ex situ characterization technologies and density functional density calculations.


 Please wait while we load your content...
Please wait while we load your content...