Regulation effects of Co2+ on the construction of a Cu–Ni(OH)2@CoO nanoflower cluster heterojunction: a critical factor in obtaining a high-performance battery-type hybrid supercapacitor†
Abstract
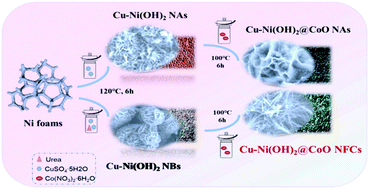
Electrode materials with a hierarchical nanostructure derived from transition metal-based compounds is an important branch of energy storage materials and have attracted widespread attention in recent years. Herein, a Cu–Ni(OH)2@CoO nanoflower cluster (Cu–Ni(OH)2@CoO NFCs) heterojunction was successfully constructed by a simple two-step hydrothermal method in the presence of Co2+. The optimized Cu–Ni(OH)2@CoO NFCs presented a high capacitive performance and outstanding cycle stability when used as a battery-type supercapacitive electrode material. In particular, an ultra-high areal specific capacitance of 5.8 F cm−2 (354.8 mA h g−1) at 1 mA cm−2 was obtained in 3 M KOH electrolyte. Even after 10 000 cycles, the capacitance still remained 98.4% of its initial value. All the experimental characterization results indicate that the excellent performance of the Cu–Ni(OH)2@CoO NFC self-supporting electrode can be attributed to the regulatory effect of Co2+ on the morphology and electronic structure, which is induced by the second hydrothermal process. More specifically, the transformations in the morphology and electronic structure will expose more active sites and accelerate charge transfer during the electrochemical reaction. Besides, the rapid oxidation reactions of multivalent transition metal ions and enhanced hydrophilicity promote the electrochemical reaction kinetics processes on the Cu–Ni(OH)2@CoO NFC electrode. This study provides a promising strategy for exploring low-cost and efficient electrode materials based on transition metal compounds for electrochemical energy storage.


 Please wait while we load your content...
Please wait while we load your content...