Neodymium-decorated graphene as an efficient electrocatalyst for hydrogen production†
Abstract
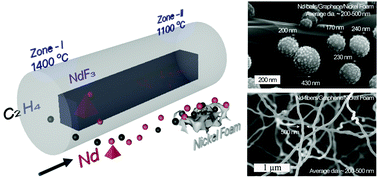
Rare earth (RE) materials such as neodymium (Nd) and others consist of unique electronic configurations which result in unique electronic, electrochemical, and photonic properties. The high temperature (>1100 °C) growth and low active surface areas of REs hinder their use as an efficient electrocatalyst. Herein, different morphologies of Nd were successfully fabricated in situ on the surface of graphene using a double-zone chemical vapor deposition (CVD) method. The morphology of the Nd material on graphene is controlled, which results in the significant enhancement of the large specific surface area and electrochemical active area of the composite material due to the spatial morphology of Nd, thereby improving the hydrogen evolution reaction (HER) performance in an alkaline medium. The significantly enhanced HER activity with an overpotential of 75 mV and a Tafel slope of 95 mV dec−1 at a current density of 10 mA cm−2 is observed in Nd-GF. Mainly, a high specific surface area of ∼2217 cm2 g−1 and the porosity of graphene play major roles in the enhancement of activity. Thus, the present work provides a new strategy for the neodymium engineering synthesis of efficient rare earth-graphene composite electrocatalysts with a high electrochemical active area.


 Please wait while we load your content...
Please wait while we load your content...