Ionic liquids as precursors for Fe–N doped carbon nanotube electrocatalysts for the oxygen reduction reaction†
Abstract
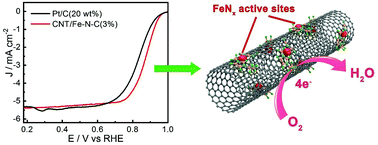
Iron and nitrogen codoped carbons (Fe–N–C) have emerged as promising noble-metal-free catalysts for the oxygen reduction reaction (ORR). However, delicate control over their structure to enhance the catalytic efficiency is still challenging. Herein, we presented the synthesis of novel ionic-liquid (IL) derived nitrogen and iron co-doped carbon nanotube (CNT) based core–sheath nanostructures that can contribute to solving these challenges associated with the ORR. These nanostructures are synthesized by the adsorption of heteroatom containing ILs on the walls of CNTs followed by carbonization. The advantage of using an IL as a nitrogen source is that the obtained catalyst has a high level of N doping and a high surface area. Electrochemical characterization revealed that the N and Fe codoped CNT based core–sheath nanostructures exhibited superior catalytic activities toward the ORR under both alkaline and acidic conditions. Particularly in alkaline solution, the CNT/Fe–N–C catalysts showed better ORR activity compared to the commercial Pt/C catalyst. We suggest that the excellent electrocatalytic performance of CNT/Fe–N–C catalysts is attributed to: (i) the synergistic effect, which provides more catalytic FeNx sites for the ORR, due to the Fe and N co-doping and (ii) the high surface area and excellent electron transfer rate arising from the IL-derived core–sheath structure.


 Please wait while we load your content...
Please wait while we load your content...