A review of Mn-based catalysts for low-temperature NH3-SCR: NOx removal and H2O/SO2 resistance
Abstract
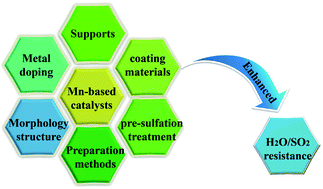
The development of high-efficiency catalysts is the key to the low-temperature NH3-SCR technology. The introduction of SO2 and H2O will lead to poisoning and deactivation of the catalysts, which severely limits the development and application of NH3-SCR technology. This review introduces the necessity of NOx removal, explains the mechanisms of H2O and SO2 poisoning on NH3-SCR catalysts, highlights the Mn-based catalysts of different active metals and supports and their resistance to H2O and SO2, and analyses the relationship between metal modification, selection of support and preparation method, morphology and structure design and SO2/H2O resistance. Given the current problems, this review points out the future research focus of Mn-based catalysts and also puts forward corresponding countermeasures to solve the existing problems.
- This article is part of the themed collection: Recent Review Articles


 Please wait while we load your content...
Please wait while we load your content...