Physicochemical implications of surface alkylation of high-valent, Lindqvist-type polyoxovanadate-alkoxide clusters†
Abstract
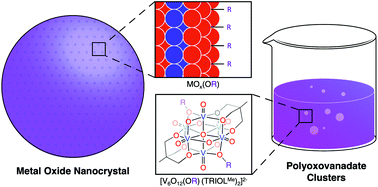
We report a rare example of the direct alkylation of the surface of a plenary polyoxometalate cluster by leveraging the increased nucleophilicity of vanadium oxide assemblies. Addition of methyl trifluoromethylsulfonate (MeOTf) to the parent polyoxovanadate cluster, [V6O13(TRIOLR)2]2− (TRIOL = tris(hydroxymethyl)methane; R = Me, NO2) results in functionalisation of one or two bridging oxide ligands of the cluster core to generate [V6O12(OMe)(TRIOLR)2]1− and [V6O11(OMe)2(TRIOLR)2]2−, respectively. Comparison of the electronic absorption spectra of the functionalised and unfunctionalised derivatives indicates the decreased overall charge of the complex results in a decrease in the energy required for ligand to metal charge transfer events to occur, while simultaneously mitigating the inductive effects imposed by the capping TRIOL ligand. Electrochemical analysis of the family of organofunctionalised polyoxovanadate clusters reveals the relationship of ligand environment and the redox properties of the cluster core: increased organofunctionalisation of the surface of the vanadium oxide assembly translates to anodic shifts in the reduction events of the Lindqvist ion. Overall, this work provides insight into the electronic effects induced upon atomically precise modifications to the surface structure of nanoscopic, redox-active metal oxide assemblies.
- This article is part of the themed collections: Celebrating International Women’s Day: Women in Nanoscience and Nanoscale 2021 Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...