Stable Li-ion storage in Ge/N-doped carbon microsphere anodes†
Abstract
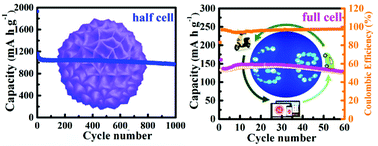
The development of environmentally benign, low-cost and high-performance Ge-based materials for lithium-ion batteries (LIBs) has remained a great challenge. Herein, the synthesis of Ge/N-doped carbon microspheres (Ge/NC) is firstly performed using N-(2hydroxyethyl)ethylenediamine (AEEA) and ethanediamine (EDA) as solvents, ligands and carbon sources. The three-dimensional Ge/NC microspheres prepared with AEEA (Ge/NC-A) are constructed from nanosheets with a thickness of about 20 nm. Such a hierarchically structured material not only allowed sufficient contact between the nanosheets and electrolyte, but also provided sufficient void space and uniform conductive sites. At the same time, N-doped carbon in the Ge/NC-A microspheres can greatly improve the electrical conductivity and the structural stability. This material exhibited a superior rate performance (633.1 mA h g−1 at 20 A g−1), favorable reversible capacity (1113.2 mA h g−1 at 0.2 A g−1) and good cycling stability (a high reversible capacity of 965.0 mA h g−1 after 1000 cycles) when examined as an anode for LIBs. A full cell was fabricated using Ge/NC-A as an anode and LiFePO4 as a cathode and delivered a capacity of 100.7 mA h g−1 after 100 cycles. Furthermore, the lithiation/delithiation mechanisms in the Ge/NC-A microspheres were revealed by in situ Raman and in situ XRD measurements, indicating that the crystalline Ge was firstly converted into amorphous Li–Ge phases and transformed into amorphous Ge during the discharge/charge process. Therefore, the repeated transition between the amorphous and crystalline phases can be avoided, thus improving the cycling stability.


 Please wait while we load your content...
Please wait while we load your content...