Unravelling the reactivity of metastable molybdenum carbide nanoclusters in the C–H bond activation of methane, ethane and ethylene†
Abstract
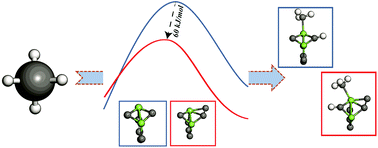
C–H bond activation steps in non-oxidative methane dehydroaromatization (MDA), constitute a key functionalization of the reactant and adsorbed species to form aromatics. Previous studies have focused on studying the energetics of these steps at the most stable active sites involving molybdenum carbide species. Herein, a different paradigm is presented via studying the reactivity of a metastable molybdenum carbide (Mo2C6) nanocluster for the C–H bond activation of methane, ethane, and ethylene and comparing it with the reactivity of the lowest energy Mo2C6 nanocluster. Interestingly, the metastable nanocluster is observed to result in a consistent reduction (by half) in the C–H bond activation barrier of the respective alkane and alkene molecules compared to the global minimum isomer. This specific metastable form of the nanocluster is identified from a cascade genetic algorithm search, which facilitated a rigorous scan of the potential energy surface. We attribute this significant lowering of the C–H bond activation barrier to unique co-planar orbital overlap between the reactant molecule and active centers on the metastable nanocluster. Based on geometrical and orbital analysis of the transition states arising during the C–H bond activation of methane, ethane, and ethylene, a proton-coupled electron transfer mechanism is proposed that facilitated C–H bond cleavage. Motivated by the high reactivity for C–H bond activation observed on the metastable species, a contrasting framework to analyze the elementary-step rate contributions is presented. This is based on the statistical ensemble analysis of nanocluster isomers, where the calculated rates on respective isomers are normalized with respect to the Boltzmann probability distribution. From this framework, the metastable isomer is observed to provide significant contributions to the ensemble average representations of the rate constants calculated for C–H bond activation during the MDA reaction.


 Please wait while we load your content...
Please wait while we load your content...