Is cannabidiolic acid an overlooked natural antioxidant? Insights from quantum chemistry calculations†
Abstract
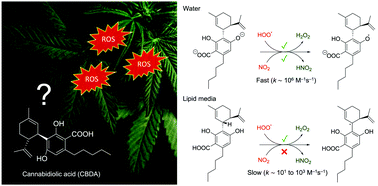
Cannabidiolic acid (CBDA) is one of the most abundant phytocannabinoids found in cannabis. This compound has demonstrated interesting biological activities, including anti-cancer, anti-inflammatory, anti-emetic, and anti-convulsant. However, due to increased emphasis on the research of its derivative, cannabidiol, its potential pharmacological effects have been hidden for years. In this contribution, a systematic study of the antioxidant capacity of CBDA was conducted using advanced quantum chemistry methods. Different reaction mechanisms at all potential positions were examined for the reaction of CBDA with the hydroperoxyl radical (HOO˙) and nitrogen dioxide (NO2). The influence of the physiological environment and the acid–base equilibrium were also taken into account. CBDA has been shown to be a good HOO˙ and NO2 scavenger in physiological polar media with rate constants of 2.40 × 106 and 1.30 × 106 M−1 s−1, respectively. This efficiency is mainly due to the increased activity of its dianionic form. When compared to some common antioxidants, the HOO˙ scavenging activity of CBDA is higher than that of Trolox and BHT (butylated hydroxytoluene), and suggests that CBDA is a promising radical scavenger, especially in polar environments.


 Please wait while we load your content...
Please wait while we load your content...