Rational design of photochromic diarylbenzene with both high photoreactivity and fast thermal back reactivity†
Abstract
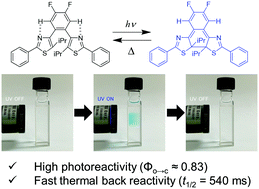
Recently, diarylbenzenes (DABs) have been developed as a new family of T-type photochromic molecules. In this work, we newly designed and synthesized DABs for the creation of molecules with both high photoreactivity and fast thermal back reactivity. Utilizing the intramolecular CH–N hydrogen bonding and the bulky substituents at the reactive carbons resulted in the enhancement of photoreactivity and the acceleration of the thermal back reaction rate. Furthermore, we demonstrated that the high photoreactivity resulted in much better coloration compared with that of the previously reported DAB even at a lower concentration. These results would not only provide a strategy for molecular design but also be useful for the development of materials with less environmental load.


 Please wait while we load your content...
Please wait while we load your content...