Application of organometallic catalysts for the synthesis of o-tolyl benzonitrile, a key starting material for sartans
Abstract
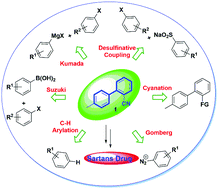
Biaryl compounds are an important class of aromatic compounds used for the synthesis of antiviral, antihypertensive, and antifungal drugs. The familiar methods for the synthesis of biaryl compounds are Pd-, Ni-catalyzed couplings reactions, such as Suzuki, Negishi and Kumada reactions. Hypertension is a medical condition in which the blood pressure against artery wall is high enough to increase the risk of heart disease and stroke. Angiotensin receptor blockers (ARBs) are one of the most effective and commonly prescribed antihypertensive agents. o-Tolyl benzonitrile (OTBN) is the common building block for the synthesis of the sartan series of drug molecules (ARBs), such as candesartan, irbesartan, losartan, tasosartan, and valsartan. The classical methods for the synthesis of OTBN are Pd-catalyzed, Ni-catalyzed Suzuki coupling reactions. The other high yield methods for the synthesis of OTBN are Ni-catalyzed Kumada coupling, and desulfinating cross-coupling using Pd-catalyst. The Ni-catalysts could be an alternative to the expensive Pd-catalyst, as cost is a factor for bulk manufacturing of this building block. Although there are few reports of OTBN synthesis using desulfinating cross coupling, this method has opened a new avenue.
- This article is part of the themed collection: 2021 Focus and Perspective articles


 Please wait while we load your content...
Please wait while we load your content...