Highly diastereoselective spiro-cyclopropanation of 2-arylidene-1,3-indanediones and dimethylsulfonium ylides†
Abstract
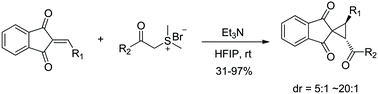
A highly diastereoselective spiro-cyclopropanation reaction of 2-arylidene-1,3-indanediones and dimethylsulfonium ylides has been developed via base-induced annulation. This efficient and simple protocol features simple operations, mild conditions and excellent functional group compatibility. A variety of structurally interesting spiro-cyclopropanes were prepared in excellent yields and diastereomeric ratios (up to 97% yield and 20 : 1 dr). Also, ring expansion of the cyclopropanation product to quickly deliver a complex indeno[1,2-c]pyridazine structure showcased an interesting application of this method.


 Please wait while we load your content...
Please wait while we load your content...