Synthesis and photophysical properties of 5-(3′′-alkyl/aryl-amino-1′′-azaindolizin-2′′-yl)-2′-deoxyuridines†
Abstract
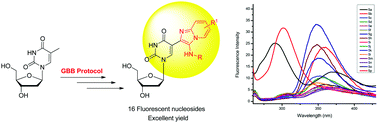
The Groebke–Blackburn–Bienayame (GBB) reaction has been used for the efficient synthesis of novel fluorescent 5-azaindolizino-2′-deoxyuridines starting from commercially available thymidine following two strategies. Thus, thymidine was converted to diacetylthymidine, which on potassium persulphate oxidation afforded 3′,5′-di-O-acetyl-5-formyl-2′-deoxyuridine. In strategy A, diacetylated 5-formyldeoxyuridine was reacted with a variety of 2-aminopyridines and alkyl/aryl isocyanides under optimized GBB reaction conditions followed by deacetylation of the resulting GBB products to afford 5-azaindolizino-2′-deoxyuridines in 83 to 95% overall yields. In strategy B, diacetylated 5-formyldeoxyuridine was first deacetylated, which on GBB reaction under standardised conditions with 2-aminopyridines and alkyl/aryl isocyanides afforded the desired 5-azaindolizino-2′-deoxyuridines in 21 to 23% overall yields, which clearly indicates that strategy A is far more efficient than strategy B. The emission spectra of the synthesized 5-azaindolizino-2′-deoxyuridines exhibited a strong band around 360 nm (excitation at 239 nm) in fluorescence studies. Photophysical studies of these nucleosides showed a high level of fluorescence with Stokes shift in the range 59–126 nm, which indicated their potential for the study of the local structure and dynamics of nucleic acids involving them.


 Please wait while we load your content...
Please wait while we load your content...