1,3,4-Oxadiazole-functionalized α-amino-phosphonates as ligands for the ruthenium-catalyzed reduction of ketones†‡
Abstract
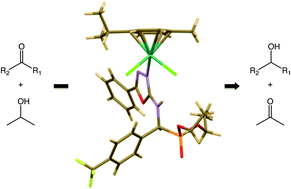
Three α-aminophosphonates, namely diethyl[(5-phenyl-1,3,4-oxadiazol-2-ylamino)(4-trifluoromethylphenyl) methyl]phosphonate (3a), diethyl[(5-phenyl-1,3,4-oxadiazol-2-ylamino)(2-methoxyphenyl)methyl]phosphonate (3b) and diethyl[(5-phenyl-1,3,4-oxadiazol-2-ylamino)(4-nitrophenyl)methyl]phosphonate (3c), were synthetized via the Pudovik-type reaction between diethyl phosphite and imines, obtained from 5-phenyl-1,2,4-oxadiazol-2-amine and aromatic aldehydes, under microwave irradiation. Compounds 3a–c underwent complexation with a ruthenium(II) precursor, selectively at the more basic nitrogen atom of the oxadiazole ring, leading to the corresponding ruthenium complexes 4a–c of the formula [RuCl2(L)(p-cymene)] (L = α-aminophosphonates 3a–c). Complexes 4a–c proved to be efficient catalysts for the transfer hydrogenation of ketones to alcohols. All new compounds were fully characterised by elemental analysis, infrared, mass and NMR spectroscopy. An X-ray structure of the α-aminophosphonate 3b was obtained and revealed the presence, in the solid state, of an infinite chain of 3b units supramolecularly interlinked. Two X-ray diffraction studies carried out on ruthenium complexes confirm the specific coordination of the electron-enricher nitrogen atom of the oxadiazole ring.


 Please wait while we load your content...
Please wait while we load your content...