Theoretical investigation of the effect of hydrogen bonding on the stereoselectivity of the Diels–Alder reaction†
Abstract
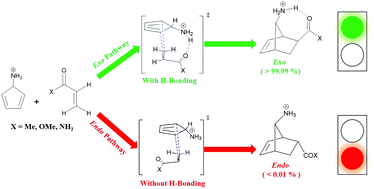
In this article, we report the computational examination of the impact of hydrogen bonding (HB) on the stereoselectivity of a series of Diels–Alder (DA) reactions. Four different types of diene/dienophile couples were studied including (a) cyclopenta-2,4-dien-1-ol and heteroatom-substituted cyclopentenes, (b) substituted cyclopentadienes and N-protonated 2,5-dihydro-1H-pyrrole, (c) furan and N-protonated 5-azabicyclo[2.1.1]hex-2-ene, and (d) N-protonated cyclopenta-2,4-dien-1-amine and α,β-unsaturated carbonyl compounds. These systems were designed such that the HB can only exist in the exo reaction pathway. The optimized structures of the transition states (TSs) and products, along with the Gibbs free energies of activation and reaction were calculated in the gas phase and dichloromethane by B3LYP/6-31G++(d,p) method. It was found that the exo adduct is the most kinetically and thermodynamically favored product for all of the DA reactions mentioned above. Moreover, the role of the HB in stabilizing the exo stereoisomer (and the corresponding TS) was much more pronounced for the reactions involving cationic species (cases b–d). In the end, a hypothetical synthetic method was proposed for obtaining exo-2-alkylbicyclo[2.2.1]heptanes. Our findings suggest that the HB can be employed as an effective interaction to control the stereoselectivity of DA reactions.


 Please wait while we load your content...
Please wait while we load your content...