Pt distribution-controlled Ni–Pt nanocrystals via an alcohol reduction technique for the oxygen reduction reaction†
Abstract
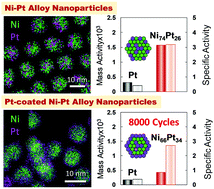
Alloying Pt with transition elements as electrodes in fuel cells has been proposed to solve the CO poisoning effect besides cost-benefit. Consequently, the use of Ni–Pt nanoparticles (NPs) has been proved to be effective among other transition metals such as Co and Fe. Though there are some reports on the synthesis of nanometer-scale particles, optimization of their composition has scarcely been reported. Thus, in this study, the influence of Pt atoms’ elemental distribution on the catalytic activities of Ni–Pt alloy NPs was investigated by measuring the oxygen reduction reaction (ORR) in acidic medium. Ni–Pt NPs with Pt concentrations between 7 and 73 at% were synthesized by an alcohol reduction method via the oleylamine complexation of Pt for controlling the size, shape and composition. Ni74Pt26 alloy NPs exhibited the highest catalytic performance, and their mass and specific activities were about 5.1 and 8 times, respectively, that of the commercial Pt catalyst. Moreover, in order to enhance the stability and durability of the particles, Pt-coated Ni74Pt26 alloy NPs were also successfully synthesized by modifying the process to facilitate the heterogeneous nucleation of Pt on the particle surface expecting coherent and ligand effects. The activity of Pt-coated Ni74Pt26 NPs was about 2 times that of the commercially available catalyst but was almost 0.5 times that of Ni74Pt26 particles. The durability was enhanced and comparable to that of the commercial Pt catalyst, suggesting that Pt coating on Ni–Pt alloy nanoparticles could be the design criterion for a highly reactive and cost-effective catalyst.


 Please wait while we load your content...
Please wait while we load your content...