Effect of electron-withdrawing groups on molecular properties of naphthyl and anthryl bithiophenes as potential n-type semiconductors†
Abstract
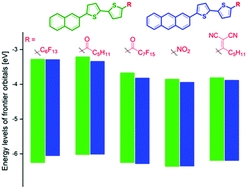
A series of ten 2-naphthyl and 2-anthrylbithiophene derivatives with electron acceptor groups were synthesised using the Negishi or Suzuki cross-coupling reaction as a key step. We present a comparison of theoretical and experimental values of the LUMO and gap energies of these derivatives and the effect of the various electron-withdrawing groups on their optical and electrochemical properties. DFT-calculated frontier orbital energy differences have shown a trend following the experimentally determined values. The participation of the electron-withdrawing group in π-conjugation decreases the LUMO level and narrows the energy gap in the order of perfluoroalkyl, acyl, perfluoroacyl, nitro ≈ dicyanovinyl in both series. TD-DFT calculations allowed better understanding of electronic transitions. X-ray structure analysis of naphthalene hexanoyl and perfluorooctanoyl derivatives revealed their herringbone or sandwich herringbone molecular packing, respectively, having a planar naphthalene-bithiophene moiety with opposite (s-trans vs. s-cis) conformation of bithiophene.


 Please wait while we load your content...
Please wait while we load your content...