Phosphorus–nitrogen compounds. Part 54. syntheses of chiral amino-4-fluorobenzyl-spiro(N/O)cyclotriphosphazenes: structural and stereogenic properties†
Abstract
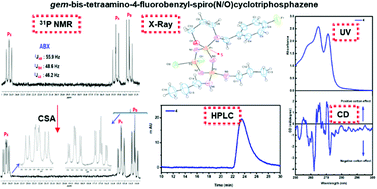
Although tetrahedrally coordinated pendant-armed heterocyclic phosphazenes are considerably stable, the stereogenic properties of their inorganic ring systems are much less explored. In the present study, 4-fluorobenzyl-spiro(N/O)tetrachlorocyclotriphosphazene (1) was obtained from the condensation reaction of hexachlorocyclotriphosphazene (trimer, N3P3Cl6) with an unsymmetrical bulky ligand, sodium 3-(4-fluorobenzylamino)-1-propanoxide (L1). In addition, the mono- (2 and 3), geminal-bis- (4 and 5) and tetraamino-4-fluorobenzyl-spiro(N/O)cyclotriphosphazenes (6 and 7) were synthesized from the Cl replacement reactions of 1 with varying stoichiometric mole ratios (1 : 1, 1 : 2, 1 : 3 and 1 : 4) of n-propylamine and pyrrolidine for the investigation of their spectral and stereogenic properties, separately. The structures of all the cyclotriphosphazenes were elucidated by elemental analysis, mass spectrometry (ESI-MS), FTIR and 1H-, 13C- and 31P NMR techniques. As expected, the geminal-bis- and mono- aminophosphazenes have one and two different chiral P-centers, respectively. The stereogenic properties of 3, 4 and 5 were examined using 31P NMR spectra recorded with the addition of optically active chiral solvating agent CSA, (R)-(+)-2,2,2-trifluoro-1-(9′-anthryl)-ethanol. The chiralities of these compounds were confirmed by circular dichroism (CD) spectra and chiral high performance liquid chromatography (HPLC) in solution, as well. The solid-state molecular and crystal structures of an enantiomer (S) of geminal-bis-propylamino-4 were characterized by the single-crystal X-ray diffraction method. In order to understand the intermolecular interactions in the crystal of 4, a Hirshfeld surface (HS) analysis was also performed.


 Please wait while we load your content...
Please wait while we load your content...