Organocatalytic aminocarbonylation of α,β-unsaturated ketones with N,N-dimethyl carbamoylsilane†
Abstract
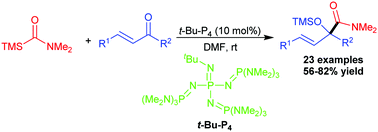
An organic superbase-catalyzed aminocarbonylation reaction of α,β-unsaturated ketones was reported. Under the catalysis of 10 mol% Schwesinger's superbase t-Bu-P4, a variety of enones and ynones reacted with N,N-dimethyl carbamoylsilane to afford α-siloxyamides or α-hydroxyamides in 56–82% yields.


 Please wait while we load your content...
Please wait while we load your content...