Synthesis and triplet sensitization of bis(arylselanyl)BOPHYs; potential application in triplet–triplet annihilation upconversion†
Abstract
Owing to the growing interest in the development of novel triplet sensitizers, the introduction of arylselanyl (ArSe) groups at the 2 and 7 positions of bis(difluoroboron)-1,2-bis((1H-pyrrol-2-yl)methylene) hydrazine (BOPHY) scaffold was investigated for producing dyes 1 and 2. Direct Se–C bond formation was effective in facilitating the intersystem crossing (ISC) channel to the corresponding triplet state, endowing them with triplet sensitization. The quantum yield (ΦΔ) of the singlet oxygen (1O2) generation was determined using diphenylisobenzofuran (DPBF), resulting in preferable ΦΔ values of 73% and 74% for 1 and 2, respectively, which were significantly higher than those of halogenated BOPHY. This was ascribed to the selenium-based heavy-atom effect on the photophysical properties of BOPHY dye, which was rationalized by estimating the spin–orbit coupling (SOC) values of the S1 →Tn transitions. The favorable ability to generate 1O2 under photoirradiation motivated us to apply the dye as a sensitizer to triplet–triplet annihilation upconversion (TTA-UC). Photoexcitation of a toluene solution of 1 and 9,10-diphenylanthracene (DPA) as the triplet energy acceptor with a 524 nm-wavelength laser allowed to detect upconverted emission with λem values of 412 nm and 430 nm, which were attributed to the DPA fluorescence. A significant anti-Stokes shift of approximately 94 nm was observed in the UC process with a 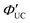 of 3.9%. This study suggests that a judicious introduction of ArSe units into chromophores provides a facile but effective strategy for designing triplet sensitizers.
of 3.9%. This study suggests that a judicious introduction of ArSe units into chromophores provides a facile but effective strategy for designing triplet sensitizers.



 Please wait while we load your content...
Please wait while we load your content...