A thiol–ene click reaction with preservation of the Si–H bond: a new approach for the synthesis of functional organosilicon compounds†
Abstract
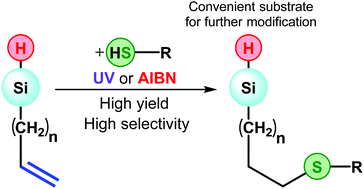
This work presents an approach for the preparation of functional hydrosilanes. The essence of the method is a thiol–ene click reaction on vinyl or allylsilanes. In this case, the Si–H bond in the substrate molecule is not affected. The reaction proceeds with radical (AIBN) or UV initiation with yields close to quantitative. This makes it possible to obtain aliphatic, aromatic, carboxyl and alkoxysilyl derivatives of the corresponding silanes. Crystalline tris(3-(phenylthio)propyl) silane was obtained and characterized for the first time. In the case when the thiol–ene reaction leads to the formation of a three-dimensional network, it was possible to obtain Si–H-containing materials – a transparent monolith and an aerogel with a density of 0.26 g ml−1.


 Please wait while we load your content...
Please wait while we load your content...