Synthesis of a light-harvesting ruthenium porphyrin complex substituted with BODIPY units. Implications for visible light-promoted catalytic oxidations†
Abstract
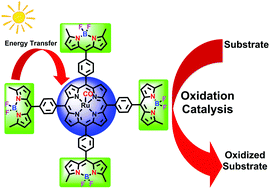
A light-harvesting ruthenium porphyrin substituted covalently with four boron–dipyrrin (BODIPY) moieties has been synthesized and studied. The resulting complex showed an efficient decarbonylation reaction predominantly due to a photo-induced energy transfer process. Chemical oxidation of the ruthenium(II) BODIPY–porphyrin afforded a high-energy trans-dioxoruthenium(VI) species that is one order of magnitude more reactive towards alkene oxidation than those analogues supported by conventional porphyrins. In the presence of visible light, the ruthenium(II) BODIPY–porphyrin displayed remarkable catalytic activity toward sulfide oxidation and alkene epoxidation using iodobenzene diacetate [PhI(OAc)2] and 2,6-dichloropyridine N-oxide (Cl2pyNO) as terminal oxidants, respectively. The findings in this work highlight that porphyrin–BODIPY conjugated metal complexes are potentially useful for visible light-promoted catalytic oxidations.


 Please wait while we load your content...
Please wait while we load your content...