Phenalenyl based platinum anticancer compounds with superior efficacy: design, synthesis, characterization, and interaction with nuclear DNA†
Abstract
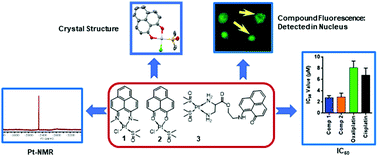
Significant efforts have been made to develop new platinum anticancer compounds since the discovery of cis-platin; however, non-specific toxicity or loss of efficacy remain some of the major challenges in this area of platinum drug discovery. Newly developed platinum compounds, structurally distinct from the current molecules, imparting efficacy at lower concentrations than the current drugs, and interacting with DNA leading to cell death, may prove beneficial. In the current study, we designed, synthesized, and characterized three phenalenyl based platinum chloride compounds (1, 2, and 3) with the goal that a labile Pt–Cl bond with the planar structure of the phenalenyl moiety would enhance their interaction with DNA, leading to improved efficacy. In addition, it is assumed that the fluorescent properties of these compounds would facilitate mechanistic investigation. The crystal structure of compound 1 demonstrates a perfectly planar structure with a single Pt–Cl bond. In vitro cell viability studies on cancer cell lines, including lung, colorectal, breast, and osteosarcoma, revealed a superior efficacy for compounds 1 and 2, relative to platinum drugs in clinical use. Localization studies utilized the strong fluorescence of compound 3 to investigate the mechanism of action, revealing its interaction with DNA, leading to cell death. This study enriches the arsenal of potential platinum-based anti-cancer therapeutics and provides an easy-to-use tool for the mechanism-of-action studies of these compounds.


 Please wait while we load your content...
Please wait while we load your content...