Unique reactivity of an α-ketiminopyridine ligand with metal–alkyls: Synthesis and ROP of ε-caprolactone†
Abstract
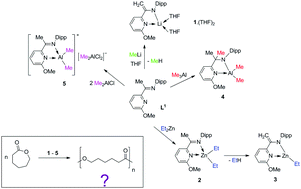
The reaction of an α-ketimininopyridine ligand 2-((Me)C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N(C6H3-2,6-iPr2))-6-(OMe)C5H3N (L1) with metal–alkyls, such as MeLi, Et2Zn, Me3Al and Me2AlCl, was studied. The reaction of L1 with MeLi led exclusively to the formation of [2-((H2C)C–N(C6H3-2,6-iPr2))-6-(OMe)C5H3N]Li·(THF)2 (1·(THF)2) with a deprotonated ketimine methyl group as a product of methane elimination. The treatment of L1 with Et2Zn provided, at the first stage, only complex [2-((Me)C
N(C6H3-2,6-iPr2))-6-(OMe)C5H3N (L1) with metal–alkyls, such as MeLi, Et2Zn, Me3Al and Me2AlCl, was studied. The reaction of L1 with MeLi led exclusively to the formation of [2-((H2C)C–N(C6H3-2,6-iPr2))-6-(OMe)C5H3N]Li·(THF)2 (1·(THF)2) with a deprotonated ketimine methyl group as a product of methane elimination. The treatment of L1 with Et2Zn provided, at the first stage, only complex [2-((Me)C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N(C6H3-2,6-iPr2))-6-(OMe)C5H3N]ZnEt2 (2). The nonstability of 2 led to ethane elimination along with the deprotonation of a ketimine methyl group, which further yielded [2-((H2C)C–N(C6H3-2,6-iPr2))-6-(OMe)C5H3N]ZnEt (3). In contrast, Me3Al reacted with L1 in a carboalumination fashion and [2-((Me)2C–N(C6H3-2,6-iPr2))-6-(OMe)C5H3N]AlMe2 (4) was isolated. In the case of Me2AlCl, an ionic species {[2-((Me)C
N(C6H3-2,6-iPr2))-6-(OMe)C5H3N]ZnEt2 (2). The nonstability of 2 led to ethane elimination along with the deprotonation of a ketimine methyl group, which further yielded [2-((H2C)C–N(C6H3-2,6-iPr2))-6-(OMe)C5H3N]ZnEt (3). In contrast, Me3Al reacted with L1 in a carboalumination fashion and [2-((Me)2C–N(C6H3-2,6-iPr2))-6-(OMe)C5H3N]AlMe2 (4) was isolated. In the case of Me2AlCl, an ionic species {[2-((Me)C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N(C6H3-2,6-iPr2))-6-(OMe)C5H3N]AlMe2}+ {Me2AlCl2}− (5) was formed as a result of a spontaneous dissociation of Me2AlCl initiated by the ligand L1. Since compounds 2–5 contain a metal–alkyl fragment, they were used as pre-catalysts in ROP of ε-caprolactone, as well as compound 1·(THF)2.
N(C6H3-2,6-iPr2))-6-(OMe)C5H3N]AlMe2}+ {Me2AlCl2}− (5) was formed as a result of a spontaneous dissociation of Me2AlCl initiated by the ligand L1. Since compounds 2–5 contain a metal–alkyl fragment, they were used as pre-catalysts in ROP of ε-caprolactone, as well as compound 1·(THF)2.


 Please wait while we load your content...
Please wait while we load your content...