Abstract
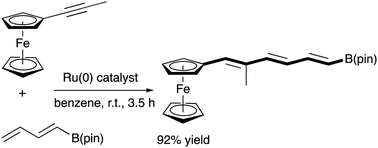
The first broadly applied strategy for the cross-dimerisation of an internal alkynylferrocene (1) with conjugated dienes (2) is revealed. The Ru(0)-catalysed cross-dimerisation of 1-propynylferrocene, FcC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CMe (1a), with (E)-butadien-1-ylboronic acid pinacolato ester, CH2
CMe (1a), with (E)-butadien-1-ylboronic acid pinacolato ester, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–CH
CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHB(pin) (2g), yields a conjugated triene, (1E,3E,5E)-FcCH
CHB(pin) (2g), yields a conjugated triene, (1E,3E,5E)-FcCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CMe–CH
CMe–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–CH
CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHB(pin) (3ag), in a 92% yield. Subsequently, the Pd(II)-catalysed cross-couplings of 3ag with a series of aryl iodides yields corresponding conjugated polyenes, such as (1E,3E,5E)-FcCH
CHB(pin) (3ag), in a 92% yield. Subsequently, the Pd(II)-catalysed cross-couplings of 3ag with a series of aryl iodides yields corresponding conjugated polyenes, such as (1E,3E,5E)-FcCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CMe–CH
CMe–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–CH
CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHAr. The photo-electronic properties of the ferrocenyl conjugated polyenes are evaluated and their electronic properties are also evaluated by time-dependent density-functional theory calculations.
CHAr. The photo-electronic properties of the ferrocenyl conjugated polyenes are evaluated and their electronic properties are also evaluated by time-dependent density-functional theory calculations.
- This article is part of the themed collection: Boron & Beyond - in celebration of Todd Marder


 Please wait while we load your content...
Please wait while we load your content...