PdII- and PtII-mediated coupling of aryl isocyanides with N-heterocyclic thiones†
Abstract
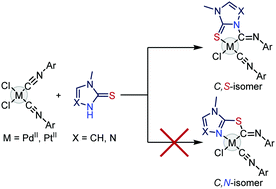
The first example of the addition of an ambident nucleophile's endocyclic center to a coordinated isocyanide is reported, namely, the PdII- and PtII-mediated reaction of aryl isocyanides with N-methylimidazole- and N-methyltriazole-2-thiones resulting in C,S-chelated deprotonated diaminocarbene complexes. The obtained complexes were isolated in good yields (88–95%) and characterized by elemental analysis (C, H, N), high-resolution mass spectrometry, and IR, 1D (1H, 13C, 195Pt) and 2D (1H–1H COSY, 1H–1H NOESY, 1H–13C HSQC, 1H–13C HMBC) NMR spectroscopies, as well as by single-crystal X-ray diffraction for the four complexes. The favorability of the resulting regioselectivity was confirmed by DFT calculations.


 Please wait while we load your content...
Please wait while we load your content...