Bimetallic RuPd nanoparticles in ionic liquids: selective catalysts for the hydrogenation of aromatic compounds†
Abstract
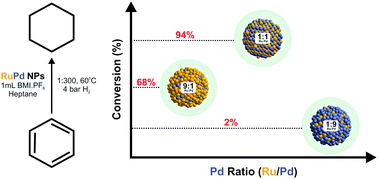
Bimetallic RuPd nanoparticles (NPs) immobilized in ionic liquids (ILs) were shown to be a highly active medium for the selective hydrogenation of benzene and phenol under mild conditions (4 bar H2, 60 °C) in a biphasic system (n-heptane/IL). The equimolar combination of Ru and Pd into a bimetallic particle generated a synergistic catalyst that allowed the selective production of cyclohexane (>99% selectivity, 94% conversion) and cyclohexanol (99% selectivity, >98% conversion) from the reduction of benzene and phenol, respectively. Moreover, the catalytic results revealed that the activity and selectivity are dependent on the Ru : Pd ratio into the bimetallic NPs.


 Please wait while we load your content...
Please wait while we load your content...