Surface modification for improving the photoredox activity of CsPbBr3 nanocrystals†
Abstract
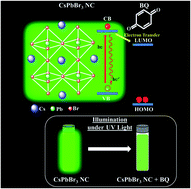
In recent years inorganic lead halide perovskite nanocrystals (PNCs) have been used in photocatalytic reactions. The surface chemistry of the PNCs can play an important role in the excited state interactions and efficient charge transfer with redox molecules. In this work, we explore the impact of CsPbBr3 nanocrystal surface modification on the excited state interactions with the electron acceptor benzoquinone (BQ) for three different ligand environments: as oleic acid/oleylamine (OA/OAm), oleic acid (OA)/trioctylphosphine (TOP), and oleic acid (OA)/oleylamine (OAm)/trioctylphosphine (TOP) ligands. Our finding concludes that amine-free PNCs (OA/TOP capped) exhibit the best excited state interactions with benzoquinone compared to the conventional oleylamine ligand environment. The photoinduced electron transfer (PET) rate constants were measured from PL-lifetime decay measurement. The amine-free PNCs show the highest PET which is 9 times higher than that of conventional ligand capped PNCs. These results highlight the impact of surface chemistry on the excited-state interactions of CsPbBr3 NCs and in photocatalytic applications. More importantly, this work concludes that amine-free PNCs maintain a redox-active surface with a high photoinduced electron transfer rate which makes them an ideal candidate for photocatalytic applications.


 Please wait while we load your content...
Please wait while we load your content...