Synthesis of naked vanadium pentoxide nanoparticles
Abstract
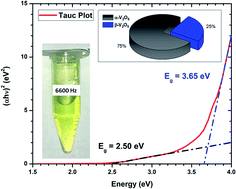
Vanadium pentoxide is the most important vanadium compound by being the precursor to most vanadium alloys. It also plays an essential role in the production of sulfuric acid as well as in metal-ion batteries and supercapacitors. In this paper, pulsed laser ablation in liquids is used to synthesize “naked” vanadium pentoxide nanostructures. The resulting particles take up “nearly-spherical” and “flower-like” morphologies, composed of α-V2O5 and β-V2O5 crystalline phases. Even “naked”, the nanostructures are stable in time with a zeta potential of −51 ± 7 mV. In order to maximize the production of vanadium pentoxide nanostructure, the optimal repetition rate was determined to be @ ∼6600 Hz when irradiating a pure vanadium target in DI-water. This corresponds to a cavitation bubble lifetime of around ∼0.15 ms. At that repetition rate, the production reached ∼10 ppm per minute of irradiation. Finally, from the characterization of the α-V2O5 and β-V2O5 nanostructures, the surface energy of each phase has been carefully determined at 0.308 and 1.483 J cm−2, respectively. Consequently, the β-phase was found to display a surface energy very close to platinum. The exciton Bohr radius has been determined at 3.5 ± 0.7 nm and 2.0 ± 0.6 nm for α-V2O5 and β-V2O5 phases, respectively.


 Please wait while we load your content...
Please wait while we load your content...